In the First Part, we discussed the processes involved in developing a Drug, from its Research to its Marketing. This part, we will discuss the Indian Pharmaceutical Industry and more.
The total size of the global pharmaceutical market is 1.2 Trillion Dollars, which are majorly contributed by three countries United States (486 Billion Dollars), China (137 billion Dollars), and Japan (86 billion Dollars). On the other hand, the Indian pharmaceutical industry is valued at 37 billion dollars as of 2018. It is home to more than 3000 pharma companies and has the highest number of US FDA approved facilities outside of the United States.
The total export by Indian pharma companies stands at 19 billion dollars out of which 6 billion alone comes from the US alone. It has proven to be the most lucrative market for global companies and continues to do so.
Increasing ANDA Filings and Approvals
Indian companies have been the major generics exporter to the US. Since 2014 many new companies have entered the US which has led to an increasing amount of ANDAs filed with the US FDA and an increasing amount of approval from the US FDA. This has been one of the reasons for the prices of drugs decreasing and the market being more competitive. In the past few years with an increasing number of companies entering the US the regulation by the US FDA has also increased.
The above chart explains the decrease in the price of a patented drug when it loses exclusivity. The price decrease in some cases has been more than 80%. The increasing number of ANDA applications for similar therapies has resulted in a highly competitive market with significant price erosion.
Concentrated US Drug Supply Chain
To get any product from a manufacturer in the hands of consumers, generally, it has to go through wholesalers and retailers. The US drug supply chain has two more links, namely pharmaceutical benefit managers and insurance companies. About a decade ago there were more than ten pharmaceutical distributors or wholesalers who contributed to more than 80% of drug purchase from the pharmaceutical manufacturers, since then the number has reduced to only three wholesalers or distributors controlling greater than 80% of the market. The increasing consolidation among buyers has led to increased bargaining power by the distributors and has put a lot of pressure on mid and small-sized generic manufacturers.
Pharmacy Benefit Managers or PBM are companies that manage prescription drug benefits on behalf of health insurers. PBM has a significant impact on determining how much of the amount would go out of the pocket of the patients and how much would a health insurer pay for a specific drug. PBM maintains a list of drugs known as formularies of covered medicines on behalf of the health insurers which determine which drugs individuals use and determine out of pocket costs. PBM’s negotiate rebates and discounts from drug manufacturers to add their drug to formularies. PBM’s then transfer this rebate and discount to insurance companies. So the higher the discounts lower the amount that a patient has to shell out of his pocket and thus lower will be the drug price for the end consumer. How much PBM’s transfer to insurance companies and how much they keep for themselves is not disclosed publically.
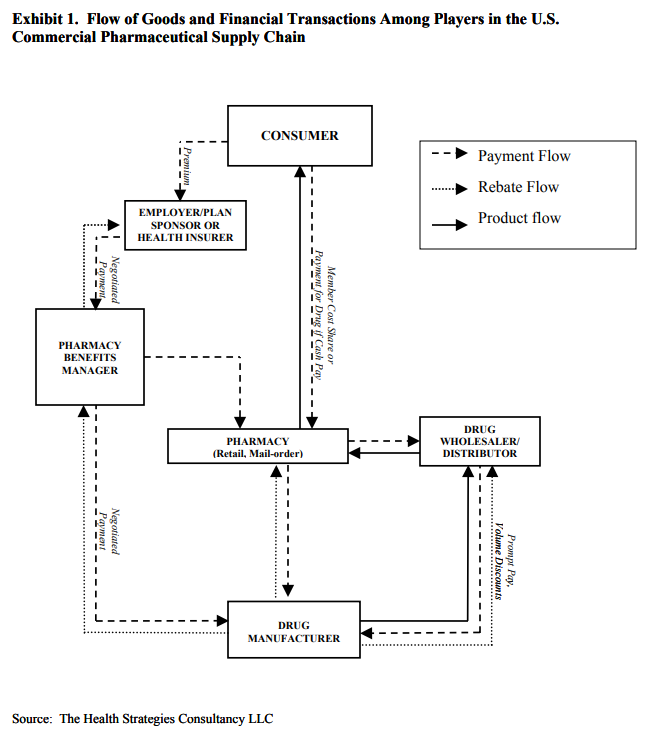

The pressure from the consolidation of the supply chain has negatively affected the Indian pharma companies in the past few years coupled with an increasing number of ANDAs filed and increasing regulatory requirements by the US FDA has affected the pricing in the generics market.
A move to complex generics
Indian pharma companies have become major generic manufacturers around the globe in the past decade. They have been the low-cost producers of generics, with ANDA filings under different therapeutic segments, but as the sector evolves, the companies also need to move up the ladder. With increasing regulation and increasing branded and unbranded generics supply, there is a need to move to a niche segment which is complex generics.
Complex generics involve a complex formulation, complex active pharmaceutical ingredient, complex drug delivery system, or an alternate drug delivery system, complex drug-device combination. One of the blockbuster drugs Advair patented by GlaxoSmithKline (GSK) had been a hot seller since 2001 its patent expired in 2010 wherein generic manufacturers entered the market. Copying the actual drug isn’t the issue. The problem seems to be that other companies can’t figure out how to copy the inhaler, a new therapeutic entity on which the company received the patent until 2016. The companies weren’t able to figure out how to deliver the drug through the inhaler until Jan 2019, only until Mylan, a US-based generic pharma company got the approval.
Suppose an X drug which has to be taken 6 times a week, twice a day and another company comes up with an alternative wherein the drug is to be taken just three times a week and once a day, so company entering such segments and modifying the existing drugs would ease them of the price pressure they are facing currently in the simple generics segment. Complex drugs are typically used to treat complex or rare conditions and chronic diseases. They are complex to manufacture and involve high-end research and high upfront investment towards product development and marketing. Many companies view it as a lucrative option but others seem to review their decisions given the impact on the profitability.
Drug Price Control Order – 2013
The Government of India enacted the Drug Price control Order (DPCO) in 1955, which was later amended in 2013. This regulation ensures the availability of essential drugs to the public at a controlled price. India has one of the lowest drug prices, but the out of pocket expenditure on healthcare remains high, and many Indians continue to be deprived of life-saving drugs. The list of the Drugs that come under the price control is provided by the National Pharmaceutical Pricing Authority. It calculates the ceiling price of drugs by taking into account the prices of drugs from different brands. National Pharma Pricing Authority (NPPA) creates a list of medicines and updates it known as National list of Essential Medicines(NLEM) under the provisions of DPCO 2013. This list includes all the drugs that are monitored and controlled by the regulator NPPA. The ceiling price is the maximum price of the drug as fixed by NPPA. DPCO puts an upper limit on margins of wholesalers and retailers to 8% and 16% respectively. The DPCO follows a market-based-pricing mechanism. The ceiling price is calculated based on the simple average price of all brands having at least 1 per cent market share of the total market turnover of that drug.
There were 348 drugs whose price was regulated by DPCO in the year 2013. This has increased to more than 800 in the year 2019. Large Indian pharma companies have been operating under Branded generics. Here, a company needs to spend on creating a brand, marketing the drug through field force or medical representatives, and having a sales force. Now, when the government ceils the price of any drug, it becomes unviable for some of the companies to incur these expenses.
Conclusion
The US pharma market has been one of the important factors for the growth of Indian pharma companies, Increasing approvals and regulations by US FDA and decreasing prices and consolidation of the supply chain has taken away the growth that the pharma companies were enjoying. The US Generics space is in the range of $70-$75 billion and hasn’t grown much. It is quite evident that Indian companies need to get into niche segments like complex generics and biosimilars. It is estimated that about $100 Billion worth of drugs are going to off-patent and about two-thirds of it will be in the complex generics space. The other major market for Indian companies being India which has grown at 7-8% for the past 5 years. The Indian pharma market has been hedging companies from the lack of growth from the US market and many companies have been concentrating on the home market and other emerging markets.



